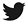3V Neil Sirolimus Drug Eluting Stent is based on L605 Cobalt Chromium platform and a biodegradable polymer. The Sirolimus used is US FDA approved and is completely released in 10 to 12 weeks. The unique formulation of PLA completely degrades into carbon dioxide and water in 10 to 13 weeks. The Cobalt Chromium platform is made in Germany in a US approved site. The Cobalt Chromium platform has a hybrid design, and the expansion happens from either side of the balloon, the stiffness of the strut is 73 µm and it is one of the lowest in this segment. The proximal shaft is PTFE coated to enhance push ability. The distal shaft is coated with unique Cath silk, hydrophilic coating that enhance cross ability and low friction response to the vessel.
Technical Specifications
| Stent Material | Cobalt Chromium L605 |
| Strut Thickness (µm) | 73 |
| Drug | Sirolimus |
| Polymer | PLA/PLGA |
| Loading dose | 1.4 micro gm/sq mm |
| Drug Elution | 90% in 4-6 weeks |
| Crossing Profile (mm.): • 2.25mm diameter • 2.50 mm diameter • 3.00 mm diameter • 3.50 mm diameter • 4.00 mm diameter |
0.87+- 0.05 0.90 +- 0.05 0.95+-0.05 1.02 + - 0.06 1.10 + - 0.08 |
| Stent Diameters (mm) | 2.25 - 4.00 |
| Stent Lengths (mm) | 8 - 40 |
| Average Foreshortening (%) | < 10% |
| Average Recoil | 7% |
| Guide Catheter Compatibility (Fr):
• 2.25 - 4.00 mm |
5 |
| Nominal Pressure (bar) | 8 |
| Rated Burst Pressure (bar): • 4.0mm (diameter) with lengths > 20mm • 4.0mm (diameter) with lengths ≤ 20mm • Balloons below 4mm (diameter) |
14 16 16 |
Ordering Information
| Length in mm | Balloon Diameter (mm) | |||||
|---|---|---|---|---|---|---|
| 2.25 | 2.50 | 2.75 | 3.00 | 3.50 | 4.00 | |
| 8 mm | N108 | N208 | N308 | N408 | N508 | N708 |
| 12 mm | N112 | N212 | N312 | N412 | N512 | N612 |
| 16 mm | N116 | N216 | N316 | N416 | N516 | N616 |
| 20 mm | N120 | N220 | N320 | N420 | N520 | N620 |
| 24 mm | N124 | N224 | N324 | N424 | N524 | N624 |
| 28 mm | N128 | N228 | N328 | N428 | N528 | N628 |
| 32 mm | N132 | N232 | N332 | N432 | N532 | N632 |
| 36 mm | N136 | N236 | N336 | N436 | N536 | N636 |
| 38 mm | N138 | N238 | N338 | N438 | N538 | N638 |
| 40 mm | N140 | N240 | N340 | N440 | N540 | N640 |